Summary of host-range testing Oberea shirahatai; a candidate biocontrol agent for Japanese honeysuckle 2013

Oberea shirahatai larvae.
O. shirahatai larvae are adapted to living inside stems and are incapable of dispersing from the plant on which the adult female beetle oviposits. For this reason, oviposition tests are critical for determining the risk of non-target attack. Larval development was quantified only for species that were oviposited on during the oviposition tests.
Choice oviposition tests using cut stems
Between 17th June and 1st July 2013 choice tests were set up using c. 30 cm cut stem material. Four adult Oberea shirahatai were placed in the cages for the first 4 replicates, 6 were added to the final two replicates. Japanese honeysuckle Lonicera japonica plants were included in all 6 replicates and different combinations of eight test plant species were also included in each replicate, so that there were nine plant species in each replicate. The test plant species used were: Abelia sp. (replicates 1,4,6); Lonicera ×americana (“American honeysuckle”; replicates 2,3,4,5,6), Lonicera ×heckrottii (“gold flame honeysuckle”; replicates 1,3,5), Lonicera periclymenum (“Graham Thomas” honeysuckle; replicates 2,4,5,6), Lonicera nitida (“Box honeysuckle”; replicates 1,2,3,4,6), Heptacodium micanoides (replicates 2,3,6), Leycesteria formosa (“Himalayan honeysuckle”; replicates 1,2,3,4,5), Morina longifolia (replicates 2,3,5,6), Symphoricarpus sp. (replicates 1,2,3,4,6), Centranthus ruber (replicates 1,2,4,5), Viburnum sp. (replicates 1,4,5,6), Weigela florida (replicates 1,3,5). The adult beetles chew holes in the stems into which they lay their eggs, but most adult feeding was on the foliage. Experiments were run for 1 week and then the number of eggs laid was counted.
 Data were analysed using Genstat, using the Generalised Linear Models option. Because the beetle numbers used in each replicate were not standardised, the number of eggs laid was divided by the number of beetles used in each replicate to give a response variable “eggs per beetle”, which was log(n+1) transformed, prior to analysis. Replicate and plant species were explanatory variables. The number of eggs laid per beetle did not vary significantly between replicates. Plant species was significant (F12,41 = 3 .11, P < 0.01; Fig. 1a.): Similar numbers of eggs were laid on Japanese (1.1 eggs/beetle/rep) and Himalayan honeysuckles (1.58 eggs/beetle/rep). The only other test plants to be oviposited on were L. ×americana (0.1 eggs/beetle/rep), Graham Thomas honeysuckle (0.375 eggs/beetle/rep) and W. florida (0.083 eggs/beetle/rep). Although many more eggs were laid on Japanese honeysuckle than these three species, the difference was only statistically significant for L. ×americana.
Data were analysed using Genstat, using the Generalised Linear Models option. Because the beetle numbers used in each replicate were not standardised, the number of eggs laid was divided by the number of beetles used in each replicate to give a response variable “eggs per beetle”, which was log(n+1) transformed, prior to analysis. Replicate and plant species were explanatory variables. The number of eggs laid per beetle did not vary significantly between replicates. Plant species was significant (F12,41 = 3 .11, P < 0.01; Fig. 1a.): Similar numbers of eggs were laid on Japanese (1.1 eggs/beetle/rep) and Himalayan honeysuckles (1.58 eggs/beetle/rep). The only other test plants to be oviposited on were L. ×americana (0.1 eggs/beetle/rep), Graham Thomas honeysuckle (0.375 eggs/beetle/rep) and W. florida (0.083 eggs/beetle/rep). Although many more eggs were laid on Japanese honeysuckle than these three species, the difference was only statistically significant for L. ×americana.
In addition to recording the number of eggs, adult feeding damage on plant foliage was recorded (0 = no feeding, 1 = light feeding; 2 = moderate feeding; 3 = heavy feeding). An analysis of feeding score as the response variable and ‘plant species’ and ‘replicate’ as the explanatory variables indicated that ‘plant species’ was significant (F12,41 = 4.42, P < 0.001; Fig. 1b.), while ‘replicate’ was not. Adult feeding occurred on more plant species, compared to oviposition, but Viburnum sp., L. ×heckrottii, Abelia sp. M. longifolia and C. ruber were not fed on at all and feeding on L. nitida was significantly lower than on the L. japonica controls (Fig 1b).
Choice-minus-host oviposition tests using cut stems
 Similar tests were performed (running concurrently with the choice test) without Japanese honeysuckle (i.e. eight plant species per replicate). Four beetles were used for reps 1& 2; 3 were used for reps 3 & 4, and six beetles were used for reps 5 & 6). Results were broadly similar to the choice host tests (there was no difference in the Log (n+1) transformed number of eggs per beetle laid between replicates, but ‘plant species’ was significant; F11,37 = 8.59, P < 0.001; Fig. 2a.), with many eggs laid on Leycesteria formosa (2.6 eggs per beetle), a few on Lonicera periclymenum (0.36 per beetle) and Lonicera ×americana (0.1 per beetle). None was laid on Weigela florida in this test.
Similar tests were performed (running concurrently with the choice test) without Japanese honeysuckle (i.e. eight plant species per replicate). Four beetles were used for reps 1& 2; 3 were used for reps 3 & 4, and six beetles were used for reps 5 & 6). Results were broadly similar to the choice host tests (there was no difference in the Log (n+1) transformed number of eggs per beetle laid between replicates, but ‘plant species’ was significant; F11,37 = 8.59, P < 0.001; Fig. 2a.), with many eggs laid on Leycesteria formosa (2.6 eggs per beetle), a few on Lonicera periclymenum (0.36 per beetle) and Lonicera ×americana (0.1 per beetle). None was laid on Weigela florida in this test.
As in the choice tests, adults fed on the foliage of more plant species than they oviposited upon. In this analysis both replicate (F5,37 = 2.99, P < 0.05) and plant species were statistically significant (F11,37 = 8.43, P < 0.001). As in the choice test, foliage of Viburnum sp., Abelia sp. Morina longifolia and Centranthus ruber were not fed on at all (Fig. 2b).
No-choice oviposition tests on whole plants
No-choice tests were performed on whole plants of Lonicera nitida (6 reps), Lonicera ×heckrottii gold flame(5 reps), Lonicera periclymenum Graham Thomas(6 reps), Leycesteria formosa (8 reps), Weigela florida (5 reps) and Japanese honeysuckle (7 reps). Oberea shirahatai beetles were confined onto each test plant for 1 week. The number of O. shirahatai beetles added to each cage varied between 2-4 beetles for all but one Japanese honeysuckle replicate. Data were analysed using Genstat, selecting the Generalised Linear Models option with ‘plant species’ as the explanatory variable. To account for variation in beetle numbers between replicates, the number of eggs laid in each replicate was divided by the number of beetles present to calculate a response variable “Eggs per beetle”.
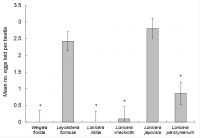 ‘Plant species’ was significant (F5,31 = 16.55, P < 0.001; Fig. 3.). No eggs were laid on L. nitida, and W. florida. Significantly fewer eggs were laid on Lonicera ×heckrottii gold flame and Lonicera periclymenum Graham Thomas versus Japanese honeysuckle: The number of eggs laid on Japanese honeysuckle control was 28.1× greater than on Lonicera ×heckrottii gold flame and 3.3× greater than on Lonicera periclymenum Graham Thomas(Fig. 3). The number of eggs laid on Japanese honeysuckle and Leycesteria formosa was not significantly different (Fig. 3).
‘Plant species’ was significant (F5,31 = 16.55, P < 0.001; Fig. 3.). No eggs were laid on L. nitida, and W. florida. Significantly fewer eggs were laid on Lonicera ×heckrottii gold flame and Lonicera periclymenum Graham Thomas versus Japanese honeysuckle: The number of eggs laid on Japanese honeysuckle control was 28.1× greater than on Lonicera ×heckrottii gold flame and 3.3× greater than on Lonicera periclymenum Graham Thomas(Fig. 3). The number of eggs laid on Japanese honeysuckle and Leycesteria formosa was not significantly different (Fig. 3).
Feeding on leaves was not quantified, but it occurred on all species except L. nitida.
Larval starvation tests using cut stems in petri dishes
While attempting to learn how to rear O. shirahatai larvae, a small-scale larval development test was set up using cut stems. The replication was very low, so the results should be treated with appropriate caution.
One larva, recently hatched from excised eggs, was used in each Petri dish. Each dish contained a cut shoot of a test plant species that was parted for a small distance down the middle of the stem and the larva placed in the gap created in the hollow centre of the stem. The larva was then able to bore into the stem material. There were four replicates for Lonicera japonica; 2 for Lonicera ×americana; 5 larvae for Lonicera periclymenum Graham Thomas and 5 larvae for Leycesteria formosa.
Shoots were checked on days 1, 5, 10, and every 5-10 days thereafter, for frass appearing out the end of the excised shoots or for the presence of dead larvae in the petri dish. Destructive sampling of the shoots only occurred when no new frass could be seen in the petri dishes.
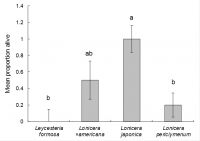 The proportions of larvae surviving in each treatment were analysed using Genstat, using the Generalised Linear Models option and selecting binomial errors and “test plant species” as an explanatory variable. After 25 days the proportion of larvae surviving significantly varied according to treatment (chi-squared = 13.393, d.f. = 3, P < 0.01), with survival on Lonicera japonica being significantly higher, compared to the other test plant species, with the exception of L. ×americana (Fig. 4).
The proportions of larvae surviving in each treatment were analysed using Genstat, using the Generalised Linear Models option and selecting binomial errors and “test plant species” as an explanatory variable. After 25 days the proportion of larvae surviving significantly varied according to treatment (chi-squared = 13.393, d.f. = 3, P < 0.01), with survival on Lonicera japonica being significantly higher, compared to the other test plant species, with the exception of L. ×americana (Fig. 4).
Beyond 25 days, “treatment effect” was no longer statistically significant due to mortality in the Lonicera japonica controls. Moreover, although no larvae survived on Leycesteria formosa,it was considered that the fleshy stems on this test species (compared to the more woody stems on the other test plants) did not last well in the Petri dishes, so the poor survival on this species was likely to have been due to declining host-plant quality.
Larval starvation tests on whole plants
The plants used in the no-choice oviposition tests on whole plants were monitored to follow the survival of the eggs laid on Lonicera ×heckrottii gold flame, Lonicera periclymenum Graham Thomas, Leycesteria formosa and the Japanese honeysuckle L. japonica controls.
Stems that had been oviposited on were monitored for signs of larval feeding (the larvae make holes in the stem, from which the periodically eject frass). The first observations were made 5 days after the oviposition test had ceased and were made at 5-10 day intervals. The single egg recorded on Lonicera ×heckrottii gold flame resulted in some initial frass production, but this did not continue beyond 25 days, indicating the larva had died.
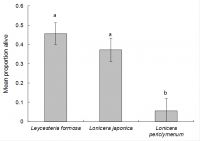 An analysis was performed to determine the proportion of larvae surviving in the other test plant species that were oviposited on (i.e. Lonicera periclymenum Graham Thomas, Leycesteria formosa and the Japanese honeysuckle controls). The proportions of larvae surviving (estimated from the number of frass holes present, divided by the initial number of eggs laid) were analysed using Genstat, using the Generalised Linear Models option and selecting binomial errors and “test plant species” as an explanatory variable. After 70 days (the most recent sample date) the proportion of larvae surviving significantly varied according to treatment (chi-squared = 7.73, d.f. = 2, P < 0.05). Survival on Lonicera periclymenum Graham Thomas (5.6%) was significantly lower than on both L. formosa (46%) and Japanese honeysuckle (37%) (Fig. 5.).
An analysis was performed to determine the proportion of larvae surviving in the other test plant species that were oviposited on (i.e. Lonicera periclymenum Graham Thomas, Leycesteria formosa and the Japanese honeysuckle controls). The proportions of larvae surviving (estimated from the number of frass holes present, divided by the initial number of eggs laid) were analysed using Genstat, using the Generalised Linear Models option and selecting binomial errors and “test plant species” as an explanatory variable. After 70 days (the most recent sample date) the proportion of larvae surviving significantly varied according to treatment (chi-squared = 7.73, d.f. = 2, P < 0.05). Survival on Lonicera periclymenum Graham Thomas (5.6%) was significantly lower than on both L. formosa (46%) and Japanese honeysuckle (37%) (Fig. 5.).
Conclusions
Plants outside of the Caprifolioideae
In the choice and choice-minus-host tests, no oviposition or adult feeding on foliage occurred on the following species: Viburnum sp. (Adoxaceae); Abelia sp. (Linnaeoideae); Morina longifolia (Morinoideae); Centranthus ruber (Valerianoideae), indicating that they are not attractive to Oberea shirahatai.
In the choice and choice-minus host oviposition tests only one egg was laid on W. florida. Given that O. shirahatai did not oviposit on W. florida in the no-choice test, the single oviposition in the choice test may indicate that oviposition on W. florida was stimulated by the presence of L. japonica test plants in close proximity (an insect’s acceptance of a non-target species can temporarily increase as a result of being stimulated by the chemistry of nearby target plants; Marohasy 1998).
The no-choice tests provided strong evidence that Weigela florida (Diervilloideae) is not attractive to ovipositing O. shirahatai and is therefore unlikely to be a field host that supports breeding populations of O. shirahatai. The fact that adult O. shirahatai did feed on W. florida leaves in the no-choice and choice tests indicates, however, that some minor spill-over adult feeding might occur on plants growing in close proximity to Japanese honeysuckle.
We conclude that larval feeding and development of O. shirahatai is confined to plants that belong to the Caprifolioideae.
Plants within the Caprifolioideae
Within the Caprifolioideae, no oviposition, and only relatively minor adult feeding was recorded on Heptacodium micanoides and Symphoricarpus sp. in the choice oviposition and choice-minus-host oviposition tests. We conclude that these species are unlikely to be field hosts that support breeding populations of O. shirahatai, but that it is possible that some adult feeding may occur on H. micanoides and Symphoricarpus sp. leaves on plants growing in close proximity to Japanese honeysuckle.
In contrast, oviposition, adult feeding and larval survival was similar on Leycesteria formosa and Japanese honeysuckle in both choice and no-choice tests, indicating that Leycesteria formosa is likely to be a suitable host for O. shirahatai in field conditions.
Ornamental Lonicera spp.
The no-choice, choice-minus host and choice oviposition tests all indicated that Lonicera nitida is not attractive to ovipositing O. shirahatai. Larval feeding tests were not done on this species because O. shirahatai females could not be induced to oviposit on L. nitida. Because O. shirahatai larvae are incapable of dispersing from the plant in which the adult female beetle oviposits, these tests indicate that L. nitida will not support breeding populations of O. shirahatai in New Zealand. Furthermore, only minor adult feeding or no adult feeding occurred on L. nitida leaves in the choice and no-choice tests, indicating that even minor spill-over adult feeding is unlikely on this species.
Only one egg was laid on Lonicera ×heckrottii gold flamein the no-choice oviposition test. Only minor adult feeding and no oviposition occurred on this species in the choice test and no adult feeding or oviposition occurred on this species in the choice-minus-host test. The larva that emerged from the egg laid in the no-choice test did not survive beyond 25 days. We conclude that that Lonicera ×heckrottii gold flame will not support breeding populations of O. shirahatai in New Zealand. Moreover, although minor spill-over attack by feeding adults is possible, even this is unlikely to occur in field conditions in New Zealand.
Oviposition on Japanese honeysuckle controls was significantly (~11×) greater than on Lonicera ×americana in the choice oviposition test indicating that this species is much less attractive to ovipositing O. shirahatai than Japanese honeysuckle. In the larval survival test, survival after 25 days was half that on Japanese honeysuckle, indicating that it may be an inferior host for larval survival, but replication in this test was too low to be certain of this. Therefore, we conclude that although the testing does not rule out the potential for oviposition on Lonicera ×americana in the field in New Zealand, non-target attack, if it occurs at all, is unlikely to be more than minor spill-over attack on plants growing in the vicinity of Japanese honeysuckle (Table 1).
Oviposition on Japanese honeysuckle controls was approximately 3 times greater than on Lonicera periclymenum Graham Thomas in both the no-choice test and in the choice test. Moreover, larval survival in the whole plant tests was a fraction (~15%) of that on Japanese honeysuckle. Therefore, we conclude that although the testing does not rule out the potential for oviposition on Lonicera periclymenum Graham Thomas in the field in New Zealand, non-target attack, if it occurs at all, is unlikely to be more than minor spill-over attack on plants growing in the vicinity of Japanese honeysuckle (Table 1).
Table 1. Relative performance on four ornamental Lonicera spp., compared to Japanese honeysuckle (e.g. the ‘relative oviposition score of 0.04 for Lonicera ×heckrottii = 0.1 eggs/beetle laid on Lonicera × heckrottii /2.693 eggs/beetle laid on L. japonica = ~0.04 in the no-choice test on whole plants). ‘Risk score’ multiplies the relative oviposition score with the larval survival score for each species (relative oviposition score × relative larval survival). A range of scores is given for Lonicera periclymenum ‘Graham Thomas’ using two estimates of larval survival from the Petri dish test and the whole plant test. A review of previous biocontrol releases indicates that there have been no cases of weed biocontrol agents attacking plants with a risk score of < 0.2 and all cases of weed biocontrol agents permanently colonising and damaging non-target plants in the field have been on plants where the risk score was > 0.55. Although a risk score < 0.2 does not guarantee non-target attack will not occur, it is considered highly unlikely than anything worse than minor spill-over will occur.
| Species | Relative oviposition* | Relative larval survival: Petri dish test | Relative larval survival: whole plant test | Risk score | Prediction |
| L. nitida | 0.00 | - | - | 0.000 | No attack |
| L. ×americana | 0.09 | 0.5 | - | 0.045 | Minor spill-over possible, but unlikely |
| L. ×heckrottii ‘gold flame’ | 0.04 | - | 0.00 | 0.000 | No attack |
| L. periclymenum ‘Graham Thomas’ | 0.31 | 0.2 | 0.15 | 0.046-0.062 | Minor spill-over possible, but unlikely |
*Calculated from the no-choice test on whole plants, except L. ×americana for which the relative scores in the choice test were used
Marohasy, J. (1998) The design and interpretation of host-specificity tests for weed biological control with particular reference to insect behaviour. Biocontrol News and Information, 19, 13N-20N.
