Data Diving Provides Pearls of Wisdom
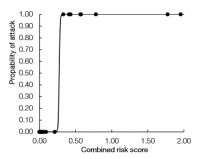
Threshold for expected non-target attack using no-choice larvalsurvival and oviposition tests as a combined risk score.
Being able to accurately predict the likely field host-range of potential biocontrol agents is critical.
Quite often when testing new agents indoors we get a low level of attack on a few non-target plants. We then need to determine whether this attack is due to laboratory conditions, and would never occur in the field, or a real risk. The best way to resolve this question currently is to undertake more natural field tests in the native range, but this is not always logistically possible. We cannot always get permission to plant out the test plants if they do not already occur in the country, or get the plants to thrive. When a field test is too difficult to undertake, there is a real danger of rejecting perfectly good agents because the dilemma cannot be resolved. When there are limited candidate agents to choose from this could mean the success or failure of a project. Even when a field test can be arranged it often takes several years to get permission, make the necessary arrangements, physically set up the trial and leave it long enough to gain meaningful results. All this adds to the cost of projects and time taken to develop new agents.
So finding a better way of assessing the risk when potential agents show some ability to potentially utilise non-target plants would be helpful, and recently Quentin Paynter has been looking into whether this might be possible. Quent carefully looked back over host-testing data for New Zealand agents and compared results with actual field data to see if these could provide any insights that might help. “The answer was yes and I have been able to develop a protoype risk index, which I presented at ISBCW,” explained Quent.
So what did Quent do? He reviewed host-range-test data for 23 agents and compiled a database of plant species growing in New Zealand (native and exotic) that supported development of the agents in no-choice tests (i.e. fundamental hosts). Then he calculated relative performance scores for how well the agents did on each test plant compared with on the target weed (e.g. laid 90% fewer eggs). Next Quent consulted the literature to identify which of the fundamental hosts identified above are field hosts in New Zealand. Where the latter was unknown Quent, with help from colleagues, conducted some field surveys to look for attack. Once Quent had all the information he ran some statistical analyses on it.
“I found that when I combined no-choice larval survival and oviposition tests into a combined risk score there was a clear threshold score (0.33) below which no attack occurred in the field,” said Quent (see graph). The technique did not work so well for choice test data, which did not produce a clear threshold point for predicting target attack in the field. Quent believes this is due to choice tests being inappropriate for seed-feeders because no-choice situations can arise in the fi eld. For example the broom seed beetle (Bruchidius villosus) can emerge from hibernation before Scotch broom (Cytisus scoparius) has produced pods, but tree lucerne (Cytisus palmensis) pods are available. “No-choice tests are more appropriate for seed-feeders than choice tests,” added Quent. “When I excluded seed-feeders, the choice tests then did have a threshold score, like the no-choice tests.”
Quent reminded the ISBCW audience that this risk assessment tool is only a prototype at this stage, and would benefit from further data from overseas studies. He asked for help to do this and some offers have already been forthcoming. So it looks likely that in the near future a useful new risk assessment tool will be available to assist both scientists and regulatory bodies to assess the risk posed by new agents.
This research was funded by the Ministry of Business, Innovation and Employment as part of Landcare Research’s Beating Weeds Programme.
