What’s Bugging Our Bugs?
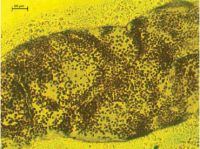
Possible symbiotic microorganism within smeared tissue of a green thistle beetle larva (400x magnification). Image - Darwin Hickman.
One possible reason for weed biocontrol agents underperforming post release is the presence of unwanted microorganisms that infect the gut and other organs. In New Zealand it has been standard practice since 1984 to routinely screen all biocontrol agents for pathogens before release, but until recently there has been no follow-up research into how effective this screening has been.
Over the past two summers Auste Cerniauskaite and Darwin Hickman, students from Birmingham University in the UK, have, as part of their studies, travelled around New Zealand exploring the health status of weed biocontrol agents, in particular comparing those released before and after 1984.
“International awareness surrounding insects hosting microorganisms has developed considerably in recent years and there are now much better DNA techniques available to aid in detecting and identifying them,” said Lindsay Smith who supervised the students during their time in New Zealand. “We are continually working to improve our screening procedures, but until now haven’t really understood how much of a role unwanted and undetected microorganisms play in the failure of released agents to control their target weeds,” he added.
First, Auste undertook a broad survey of 16 biocontrol agents (15 insect and 1 mite species) on eight weeds. These were collected from 27 sites nationwide between October 2013 and March 2014. Four of these agents had been released prior to 1984, when disease screening began: the gorse seed weevil (Exapion ulicis) released in 1931; St. John’s wort beetles (Chrysolina hyperici and C. quadrigemina – not separated to species in this study) released in 1943 and 1963, respectively; nodding thistle receptacle weevil (Rhinocyllus conicus) released in 1972; and ragwort flea beetle (Longitarsus jacobaeae) released in 1983.
On returning to the lab at Lincoln, Auste smeared the insects onto glass slides, stained them to highlight associated microorganisms, and examined the smears using high-power light microscopy. Insect pathogen specialist and microbiologist Sean Marshall from AgResearch was called in to view and comment on selected specimens of interest. Samples containing a high loading of microorganisms were assessed using PCR molecular techniques that targeted a variety of potentially pathogenic microorganisms including fungi, bacteria and Archaea (single-celled microorganisms closely related to eukaryotes).
Auste found that 15 out of the 16 biocontrol agents were free from potentially pathogenic microorganisms. The only exception was the nodding thistle receptacle weevil (R. conicus), which had some samples that were ‘hooching’ with bacteria that could be pathogens. These bacteria could be affecting weevil fecundity, larval size, or longevity and therefore their capacity to consume seeds. The bacteria, which have not yet been identified, were present in weevil specimens collected from one North Island site and three South Island sites, but were absent at other sites.
 A decision was then made to focus more fully on thistle agents when Darwin ‘picked up the reins’ a year later. He sampled 26 sites between December 2014 and March 2015 for seven insect species: the nodding thistle receptacle weevil, crown weevil (Trichosirocalus horridus) and gall fly (Urophora solstitialis); Californian thistle leaf beetle (Lema cyanella) and gall fly (U. cardui); Scotch thistle gall fly (U. stylata); and the green thistle beetle (Cassida rubiginosa). All except the receptacle weevil were screened for potentially pathogenic microorganisms prior to release in New Zealand. Some limited releases of the Californian thistle leaf beetle and gall fly were made prior to 1984 but did not result in establishment. Subsequent efforts to establish both species were made in the 1990s using screened populations, but still with limited success. Despite thousands of leaf beetles being released, they only established at one site near Auckland. The gall fly also remains extremely rare as stock will happily eat galled thistles, and Darwin was unable to collect any usable samples.
A decision was then made to focus more fully on thistle agents when Darwin ‘picked up the reins’ a year later. He sampled 26 sites between December 2014 and March 2015 for seven insect species: the nodding thistle receptacle weevil, crown weevil (Trichosirocalus horridus) and gall fly (Urophora solstitialis); Californian thistle leaf beetle (Lema cyanella) and gall fly (U. cardui); Scotch thistle gall fly (U. stylata); and the green thistle beetle (Cassida rubiginosa). All except the receptacle weevil were screened for potentially pathogenic microorganisms prior to release in New Zealand. Some limited releases of the Californian thistle leaf beetle and gall fly were made prior to 1984 but did not result in establishment. Subsequent efforts to establish both species were made in the 1990s using screened populations, but still with limited success. Despite thousands of leaf beetles being released, they only established at one site near Auckland. The gall fly also remains extremely rare as stock will happily eat galled thistles, and Darwin was unable to collect any usable samples.
Darwin used the same techniques as Auste in the laboratory. In total, he found seven distinct potentially pathogenic microorganisms using PCR, six of which were bacteria and the remaining one, which was found in the green thistle beetle, was thought initially to be a Cryptococcus yeast. “We are currently working with colleague Mike Cripps from AgResearch and Hassan Salem of the Max Planck Institute in Germany to investigate this further,” said Simon Fowler, who leads the Beating Weeds research programme. “Independently, research at the Max Planck has revealed that the green thistle beetle from Europe and USA has a common, previously undescribed bacterium as a probable gut symbiont. It is uncertain at present whether we have found the same symbiont, or something completely different,” he added. Another complication is that even if we find something in our biocontrol agents, we can’t be sure whether it was imported with the agent or is something the insects have picked up from the New Zealand environment.
Overall results so far suggest no significant difference in the presence of potentially pathogenic microorganisms in insects released before or after disease screening began, suggesting that the unscreened insects were fortunately clean when released. It may be significant that the one agent found infected with high levels of a potential pathogen, the receptacle weevil, was released without pathogen screening, however more unscreened agent species need to be tested. Although the microorganism in the receptacle weevil may be reducing its performance, it is clear that underperformance on other species is not a result of pathogens suggesting that other factors, such as environmental or abiotic conditions, are possibly more important. For example, the Californian thistle leaf beetle that has only established at one site had no detectable pathogens associated with it. “Over the past decade, we have encountered a number of pathogens in biocontrol agents that had to be eliminated in containment, as they were quite damaging to their hosts,” said Simon. “We have had microsporidia (a single-celled intercellular parasite) in heather beetles and barberry weevils, gregarines (a common protozoan parasite) in the tradescantia beetles, as well as “Ca. Liberibacter” in broom psyllids. Ensuring that the insects we released were free from these potential pathogens has meant costly delays to several of our programmes as we have had to work out how to do this,” said Simon. “Fortunately not all of the microorganisms we detect, e.g. with PCR, appear to be harmful to the insects, and some might even be symbiotic with a mutually beneficial relationship,” he added. Sorting out whether detected microorganisms are potential pathogens (which may cause agent underperformance), or beneficial symbionts (without which the agent may also underperform), is going to be a future research challenge.
“Auste and Darwin have both returned to the UK to complete their studies but their reports provide a valuable contribution to our work and a good foundation for further research,” said Lindsay. One of the long-term aims of this research is to improve screening methods for microorganisms that may be pathogenic, so they can be eliminated more cost-effectively. For example, if the frass (faeces) of individual insects collected from the field in the native range can be tested for potentially pathogenic microorganisms, unclean insects can be rejected at an early stage. “If the presence of pathogens is eliminated from the equation, and is not influencing the effectiveness of the insects, it takes out one of the potentially big performance variables,” Lindsay concluded.
This project is funded by the Ministry of Business, Innovation and Employment as part of Landcare Research’s Beating Weeds programme. We are grateful to the many regional council staff who assisted by providing specimens, access to field sites or useful advice.
