New ways of removing excess nitrogen from agricultural ecosystems

Fertiliser truck. Image - John Hunt
Adding fertiliser nitrogen (N) to soil is a common agricultural practice worldwide, but less than 20% is actually harvested in plants. The remaining excess nitrogen from fertiliser and livestock excreta is reactive and can have undesirable environmental consequences, such as increasing greenhouse gas emissions and detrimentally affecting aquatic ecosystems. A group of New Zealand and USA scientists, working together since 2014, recently discovered a previously unknown pathway for removing excess reactive N in soil.
They found that reactive N could be chemically converted to unreactive di-nitrogen (N2) gas without forming the greenhouse gas nitrous oxide (N2O) as an intermediate product. Our atmosphere is 78% N2, so when reactive N becomes N2 in the atmosphere, this is considered a permanent sink. Atmospheric N2 is not reactive and is not a greenhouse gas. The international team have applied new approaches and techniques developed by Dr Andrew McMillan (Landcare Research) and Professor Craig Tobias (University of Connecticut) to confirm chemical formation of hybrid N2 under standard conditions of temperature and pressure. Hybrid N2 is formed when organic N and inorganic N are combined. Current models of the nitrogen cycle indicate hybrid N2 is evidence of biological production by very specific bacteria or fungi in the absence of oxygen. However, this new pathway shows hybrid N2 formed in the absence of biology and in the presence of oxygen. This exciting discovery may help explain gaps in the global nitrogen budget and provide new opportunities for mitigating excess reactive N in the environment. Preliminary data from investigations at the Virginia Institute of Marine Sciences provide additional evidence that hybrid N2 is formed abiotically, particularly for deep sediments. These results are significant, as they demonstrate the potential for abiotic N2 formation worldwide.
These research finding were made possible by a new analytical instrument capable of measuring atmospheric concentrations of both N2 and N2O. This instrument, owned by Landcare Research, is the only one of its type in New Zealand (Fig. 1). The technology facilitates new advancements in the field of soil N cycling in New Zealand that were not possible previously. Analytical instruments at the University of Connecticut, tuned to detect isotopes of N2, utilised microbiological protocols developed by the New Zealand team (Figs 2 & 3) to provide independent data. An international team effort was then able to distinguish chemical formation of hybrid N2 as completely independent of N2O – a finding that has not been reported until now.
The team is now developing proposals for further funding that will allow them to investigate on-farm applications for transforming excess N from soil and water into unreactive atmospheric N2 gas without producing N2O. This may allow scientists to develop options to manage the fate of agricultural N while avoiding greenhouse gas emissions.
Acknowledgements: This work was partially funded by a USDA-NIFR grant [2014-67019-21614]; New Zealand's Ministry of Business Innovation and Employment, Royal Society of New Zealand International Travel Programme; and New Zealand Ecosystems and Global Change Fund. The authors are grateful to Veronica Rollinson at UConn, Trish McLenachan at Massey University, and Megan Peterson and Landcare Research Auckland for invaluable technical assistance.
REBECCA PHILLIPS, ANDREW McMILLAN, GWEN GRELET, BEVAN WEIR, SUJATHA SENANAYAKE AND THILAK PALMADA – LANDCARE RESEARCH
BONGKEUN SONG – VIRGINIA INSTITUTE OF MARINE SCIENCES, USA
CRAIG TOBIAS – UNIVERSITY OF CONNECTICUT, USA
E: PhillipsR@landcareresearch.co.nz
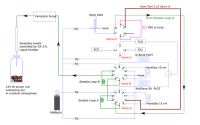
Figure 1: Schematic of the robotized instrument developed for simultaneous N2 , N2O, CO2, and O2 analyses at Landcare Research, Palmerston North.

Figure 2. Rebecca Phillips uses sterile techniques in the laboratory to quantify chemical and biological production of N2O and N2.

Figure 3. Thilak Palmada sets up the evacuation line for measurements of N2 and N2O by controlling headspace with either 100% helium or heliox (20% oxygen, 80% helium).
