The role of multiple wildlife hosts on TB persistence

Image - Caroline Thomson
Mycobacterium bovis, the causative agent of bovine tuberculosis (TB), has a very broad host range, which in New Zealand is dominated by cattle, brushtail possums, red deer, ferrets and feral pigs.
The possum has long been considered the primary wildlife maintenance host in which the disease can persist independently. Therefore, wildlife management programmes aimed at reducing TB transmission to domestic cattle and deer in New Zealand have focussed on lethal control of possums. However, there is still the occasional report of TB being found in deer, pigs, or ferrets long after the imposition of intensive possum control, sparking concerns around the combined role of other wildlife species in maintaining M. bovis and thus disease across landscapes, and the potential for spillback transmission of M. bovis to recovering possum populations.
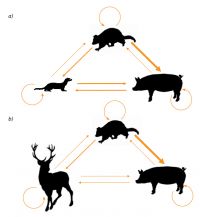
To investigate these concerns, Mandy Barron and colleagues constructed a multi-host TB model incorporating population dynamics for three host species with both intra- and inter-species M. bovis transmission. The models were parameterised for two case studies of current concern for TB management in New Zealand, namely chronic TB persistence in (i) a possum-deer-pig complex in extensive forest such as the Hauhungaroa Ranges and (ii) in a possum-pig-ferret complex in semi-arid shrub and grasslands, such as on Molesworth Station (Fig.). TB persistence in the face of ‘best practice’ possum control was evaluated from model simulations and from the contribution of the different hosts to TB persistence by removing each host species in turn from the simulations. Demographic parameter values for the different host species were readily obtained from the literature. However, disease parameter values, in particular transmission rates between host species, were largely unknown, and were derived from contact and scavenging rates or by using the ratio of home range sizes as a proxy for contact rates. Because of this uncertainty, a sensitivity analysis was done to explore how different parameter values affected modelled TB persistence.
The forest case study showed that inter-specific transmission could influence TB dynamics in possums, but this was predicted to have little effect on the success of best-practice TB control. The presence of deer had very little influence on TB dynamics in possums, supporting their long inferred status as a largely inconsequential spillover host for the disease. Also, the long-run consequences of spillback transmission to possums are minimal other than extending the duration of the possum control required. Pigs were predicted to have the most influence on possum TB prevalence, largely as a consequence of their disease amplification role. Because they are relatively short-lived, pigs did not have any great effect in extending the spillback risk to possums. Sensitivity analyses indicated these interpretations were robust to uncertainty in model parameter values.
The grassland case study showed TB could not persist in pig or ferret populations alone, but that the simultaneous presence of moderate density ferret and pig populations enabled TB to persist even when the density of possums was zero. This resulted in spillback transmission to possum populations after they had recovered from simulated control and, ultimately, TB eradication failure. The ability of ferrets and pigs to maintain TB within their collective populations was contingent upon sufficient pig-to-ferret and ferret-to-pig transmission, which was assumed to occur via reciprocal scavenging. However, TB persistence outcomes were most sensitive to these inter-specific transmission rates and unfortunately these rates are the ones the team has least information about. Simulation of population control of either pigs or ferrets to reduce their combined abundance below the threshold for disease persistence (<5 per km2) showed that, in conjunction with possum control, TB could be eradicated from an area.
This study was the first attempt to characterise multi-host TB dynamics for New Zealand wildlife and many of the disease parameter value estimates used were coarse approximations. However, the sensitivity analysis has shown which multi-host species complexes disease modellers and disease managers need to be concerned about (possums-pigs-ferrets) and which they don’t (possums-deer-pigs), as well as which parameters make the most difference to TB persistence predictions. Thus, future work should focus on the empirical estimation of ferret and pig intra- and inter-specific transmission rates to determine if multi-host dynamics could be jeopardising TB eradication programmes in semi-arid shrub and grassland habitats.
This work was funded by TBfree New Zealand and the Ministry of Business, Innovation and Employment