Estimating TB transmission rates among possums in the wild
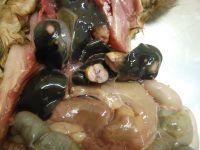
TB lesions in the liver of a possum; infecting a possum with TB. Image - Carlos Rouco
The primary wildlife reservoir of bovine tuberculosis (TB) in New Zealand is the possum, with transmission of infection from possums to livestock regarded as the largest barrier to eradicating TB from livestock.
Managing infectious diseases such as TB requires a comprehensive understanding of how the disease is transmitted from one individual to another and the rate at which it does so. However, estimating transmission rates for wild animals is particularly difficult. As a consequence, such rates are frequently estimated indirectly, e.g. as the rate required for an observed level of infection. When disease dynamics are unclear, the measurement of transmission rates from experimentally infected individuals to other susceptible individuals can provide direct estimates to confirm or question indirectly obtained values.
 To provide such a test for TB in possums, Carlos Rouco and colleagues from Landcare Research, Massey University and AgResearch have been running ‘transmission trials’ with possums in the Landcare Research Orongorongo Valley research area in the Rimutaka Forest Park. The study sought to determine the number of individuals contracting TB from experimentally infected individuals sharing their home range, and to calculate transmission rates directly from these data. Since the possum population in the study area is to be controlled (poisoned) in the very near future (and any infected possums destroyed), the team was able to introduce a strain of TB for the experimental (‘secondary’) infections that differed from local natural infections.
To provide such a test for TB in possums, Carlos Rouco and colleagues from Landcare Research, Massey University and AgResearch have been running ‘transmission trials’ with possums in the Landcare Research Orongorongo Valley research area in the Rimutaka Forest Park. The study sought to determine the number of individuals contracting TB from experimentally infected individuals sharing their home range, and to calculate transmission rates directly from these data. Since the possum population in the study area is to be controlled (poisoned) in the very near future (and any infected possums destroyed), the team was able to introduce a strain of TB for the experimental (‘secondary’) infections that differed from local natural infections.
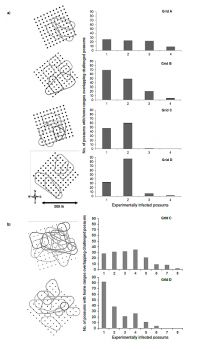
Trapping grids of 100 cage traps set at 40 m spacings (c. 13 ha) were trapped monthly to identify possum home ranges. In the first trial, four adult possums were experimentally infected on each of four such grids (two in winter (Grids C & D) and two in spring (Grids A & B) 2012; Fig. a). In the second trial, eight adult possums were experimentally infected on each of two further grids in spring 2013 (Fig.b). Six months after each experimental infection, all possums trapped on each grid were euthanased and examined for visible signs of TB and their key lymph nodes pooled for bacteriological culture to distinguish experimental from background strains of TB.
TB transmission rates from all euthanased possums were calculated for each trapping grid from the number of secondary infections detected divided by the total number of home range overlaps between experimentally challenged possums and all other possums. In the first (2012) trial there were 80–142 such overlaps on the four trapping grids, with three secondary cases of TB being detected (Fig.a). Based on this data, the possum-to-possum TB transmission rates were estimated as 0.000, 0.008, 0.004 and 0.000 on the four grids. Zero to one secondary cases therefore occurred in every 125 overlaps in home range between experimentally infected and other possums on these grids. In the second (2013) trial there were 166–182 overlaps in home range with eight secondary cases detected (Fig. b), resulting in estimated transmission rates of 0.013 and 0.003 (and approximately one to four secondary cases occurring for every 300 overlaps).
Of these six estimated transmission rates, only two were close to the level required for TB to persist in the Orongorongo Valley possum population. Based on the local possum densities on each grid, the transmission rate of 0.008 recorded in the first trial would have resulted in similar numbers of secondarily infected to experimentally infected individuals, while the rate of 0.013 in the second trial would have resulted in approximately 50% more secondarily infected possums. On the other four grids, there was insufficient transmission for the experimentally infected individuals to replace themselves.
These results have two key implications for the team’s understanding of TB dynamics in possum populations. Firstly, transmission is highly variable across both space and time. Hence, understanding the conditions that lead to high rates of transmission could potentially allow the better targeting of control efforts for TB management. Secondly, the generally low rate of transmission observed suggests that 6 months may have been insufficient to document all of the secondary cases that would have occurred. Indeed, infected possums have been documented surviving for many years, periodically relapsing with and then resolving clinical disease. Such dynamics are common for TB infection in other species (including humans), and their consideration may be important for understanding how TB persists in possum populations.
This work is part of a larger project jointly funded by TBfree New Zealand and the Royal Society of New Zealand Marsden Fund.