From fruit fly to vertebrate pest control

Fruit fly
Toxins are essential tools for vertebrate pest control because trapping and other approaches become too costly at a large scale. However, concerns over their environmental persistence and non-target impacts – particularly on pets, livestock and native species – reduce the public’s acceptance of the main toxins currently used in New Zealand.
As a consequence, new innovative technologies such as the genome mining approach highlighted in this issue of Kararehe Kino (see Hopkins et al) are being investigated as a means of developing species-selective toxins to support initiatives such as Predator Free NZ and Zero Invasive Pests. However, the normal development processes to produce registered efficacious products need to be significantly fast-tracked to get these new tools into the hands of pest managers as quickly as possible.
To achieve this, Brian Hopkins is investigating new ways of screening compounds that could accelerate product development. Traditional approaches generally consist of in vitro assays, in vitro cell culture, and enzymatic or receptor binding assays. In these assays, the chosen physiological target (an enzyme, receptor or other key protein) is presented in a way that allows high-throughput screening of compounds to identify candidates that can be developed (through extensive medicinal chemistry programmes) into useful toxins.
These assays can be extremely expensive and often don’t predict in vivo outcomes: the majority of positive hits identified through such in vitro screens are found to be ineffective in subsequent validation experiments using whole-animal models. They are also unable to determine many of the essential characteristics required for commercialisation, such as oral availability and stability. These shortcomings often result in inappropriate compounds being selected, which are a waste of money and can cause significant delays in product development.
Brian has recently come up with an approach that could overcome these shortcomings. The approach involves adapting a sophisticated in vivo fruit-fly (Drosophila melanogaster) model that has often been used for screening human therapeutic drugs, in order to provide an alternative low-cost assay to decrease the time taken to discover and develop species-selective vertebrate pest toxins.
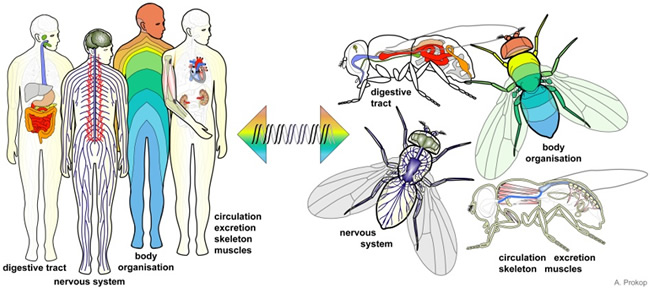
The fruit fly is a versatile model organism, which has been used in biomedical research for over a century to study a broad range of drug-related phenomena. As such it is the most extensively used and one of the best understood of all the model organisms. What makes it so useful is the surprisingly close relationship between fruit flies and mammals, with many basic biological, physiological and neurological properties being preserved and many of the control genes being common to both species. For example, 75% of the genes known to be linked to human diseases are also found in the fruit fly and can be used as targets for human therapeutic drug development. As a consequence, species-specific targets identified in a vertebrate pest as being suitable for toxin design (their ‘Achilles heel’) can be inserted into the fly’s genome with the knowledge that they will behave in a functionally similar way to those in the pest itself. Essentially, the fly will become a highly predictive in vivo surrogate pest model.
Further advantages of the fruit-fly model include its extremely low cost of maintenance, propagation and screening, and the rapidity of studies in the fly due to short generation times compared with traditional mammal-based models.
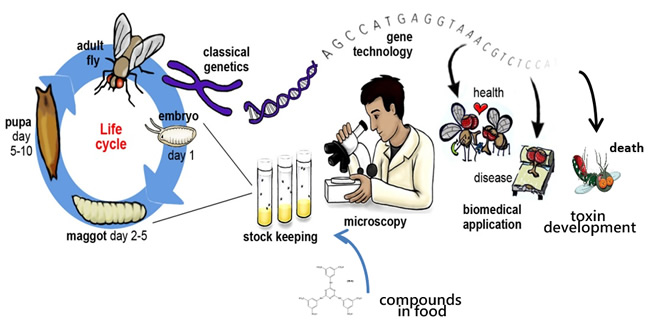
Compounds designed to target pests’ Achilles heel will be screened by administration through the fruit flies’ water supply or their food, and their lethal activity directly assessed on the assumption that compounds lethal to the fly could also be lethal to the pest species. Compounds identified this way will also have already-confirmed oral availability and metabolic stability, allowing them to be progressed significantly quicker down the developmental pathway to a usable product.
Once proven, this screening model will be readily adaptable, accelerating the discovery of selective toxins for a wide range of pest species.
Brian Hopkins (Landcare Research) hopkinsb@landcareresearch.co.nz
