Predicting the impacts of facial tumour disease on populations of Tasmanian devils

Tasmanian devil. Image - iStock.
Wildlife diseases can lead to significant losses of individuals over short periods of time and may ultimately result in extinctions either directly or indirectly by making populations more vulnerable to other threats such as predation or habitat loss. Understanding the potential impacts of disease outbreaks is thus essential for effective conservation management. When knowledge about a disease is scarce (e.g. where it has newly emerged), mathematical modelling can be used to indicate what impacts could occur based on the information available.
Tasmanian devil populations are threatened by a fatal infectious disease known as devil facial tumour disease (DFTD). The disease causes malignant facial tumours, limiting an individual’s ability to feed, and typically results in death within 6 months of infection. Signs of DFTD were first detected in north-eastern Tasmania in 1996 and the disease has since spread over most of the species’ range, leading to major population declines. This has raised questions about the potential long-term impact of DFTD on devil populations.
To gain an insight into what this impact is likely to be, and hence indicate the most appropriate strategies for managing the disease, Dan Tompkins and Amy Whitehead have been using an epidemiological mathematical modelof disease dynamics to investigate whether or not (and, if so, how) the transmission of DFTD between devils is related to population density. This is one of the key questions to ask for any wildlife disease. Many diseases are ‘density dependent’; that is, transmission rates decrease as population size decreases. Where this is the case, diseases generally ‘fade out’ as populations decline and hence are self-limiting. However, if disease transmission is independent of density, they can potentially have much greater impacts on host populations.
The model simulated a population of devils and contained information about population age and sex structure, in addition to individual infection status (Fig. 1). Such complexity is required to realistically model DFTD in devils as the disease is strongly believed to be transmitted via biting linked to breeding (i.e. male–male transmission associated with competition for mates and male–female transmission during mating).
To investigate whether DFTD transmission is density dependent or density independent, a range of different mathematical functions for the transmission process known from other diseases were modelled to assess which function (or combination of functions) best simulated disease dynamics observed in the field. The models also predicted the associated change in population size of devils over 50 years to assess the likelihood that DFTD would lead to their extinction.
Out of the transmission functions assessed, two provided the closest fit to the best available field data on disease prevalence (i.e. the proportion of infected individuals) from an infected population of devils in Freycinet National Park, Tasmania (Fig. 2); namely ‘frequency dependence’ (a density-independent function commonly associated with sexually transmitted diseases in which transmission rate is related to the proportion of infected individuals) and ‘combined frequency and density dependence’ (background density-dependent transmission occurring year round with an additional higher rate of frequency-dependent transmission during the breeding season).
Dan and Amy’s modelling exercise strongly indicates that at least some, if not all, transmission of DFTD is independent of density. As a consequence, the disease is not likely to be self-limiting through fade-out as populations decline, but could potentially cause rapid population decline (and perhaps) extinction. The model predicts that populations are likely to fall below 10% of their original size within a decade of infection being detected (Fig. 2). This conclusion reflects field observations to date. Hence, if the goal is to conserve devils in the wild, management intervention that can limit the impact of the disease in the wild is needed immediately. Longer term options such as vaccination or breeding for resistance could take well over a decade to achieve – by which time, it would most likely be too late.
This work was funded by Landcare Research Capability Funding.
Amy Whitehead & Dan Tompkins
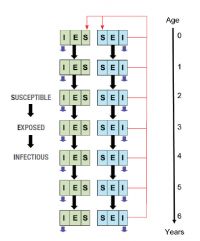
Fig. 1. Model of DFTD infecting Tasmanian devils. Green and blue compartments denote male and female animals respectively. Thick black arrows indicate the flow of animals between model compartments, red arrows indicate production of offspring by breeding adults, and short blue arrows indicate losses due to mortality (both natural and disease induced).

Fig. 2. Predicted DFTD dynamics under (a) density-dependent and (b) frequency-dependent transmission (combined density and frequency dependence not shown). The left column shows estimated DFTD prevalence (solid line) plotted against observed prevalence (points), while the right column shows predicted population trends.
